Jones Oxidation on:
[Wikipedia]
[Google]
[Amazon]
The Jones oxidation is an organic reaction for the  Jones reagent is a solution prepared by dissolving
Jones reagent is a solution prepared by dissolving
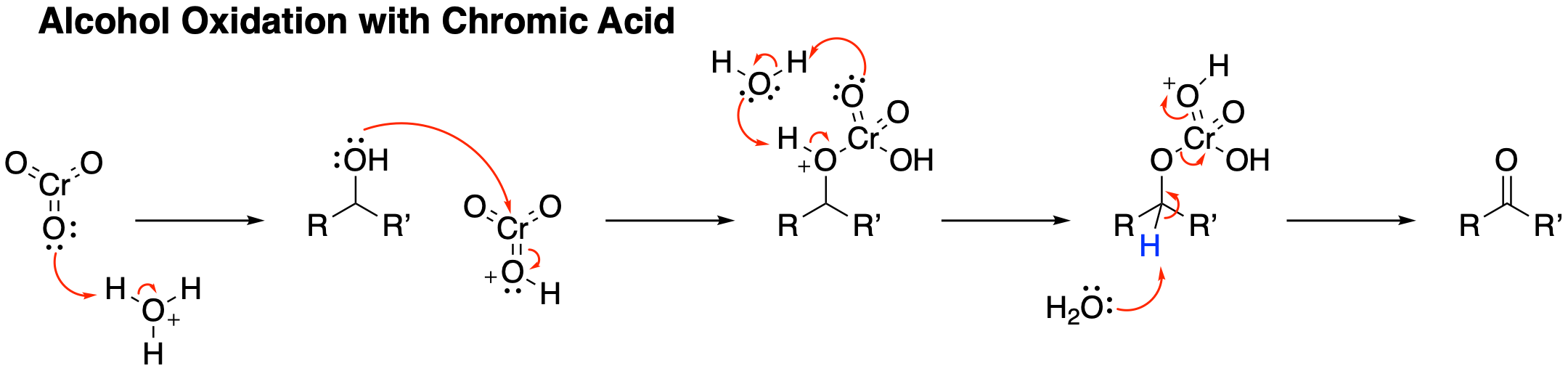 The reaction stoichiometry implicates the Cr(IV) species "CrO2OH−", which comproportionates with the chromic acid to give a Cr(V) oxide, which also functions as an oxidant for the alcohol.
The oxidation of the aldehydes is proposed to proceed via the formation of
The reaction stoichiometry implicates the Cr(IV) species "CrO2OH−", which comproportionates with the chromic acid to give a Cr(V) oxide, which also functions as an oxidant for the alcohol.
The oxidation of the aldehydes is proposed to proceed via the formation of
oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
of primary and secondary alcohols to carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s and ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent
Collins reagent is the complex of chromium(VI) oxide with pyridine in dichloromethane. This metal-pyridine complex, a red solid, is used to oxidize primary alcohols to the corresponding aldehydes and secondary alcohols to the corresponding keton ...
.
 Jones reagent is a solution prepared by dissolving
Jones reagent is a solution prepared by dissolving chromium trioxide
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.
This compound is a dark-purple ...
in aqueous sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
. To effect a Jones oxidation, this acidic mixture is then added to an acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
solution of the substrate. Alternatively, potassium dichromate
Potassium dichromate, , is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health ...
can be used in place of chromium trioxide. The oxidation is very rapid and quite exothermic. Yields are typically high. The reagent is convenient and cheap. However, Cr(VI) compounds are carcinogenic, which deters the use of this methodology.Stoichiometry and mechanism
Jones reagent will convert primary and secondary alcohols to aldehydes and ketones, respectively. Depending on the reaction conditions, the aldehydes may then be converted to carboxylic acids. For oxidations to the aldehydes and ketones, two equivalents of chromic acid oxidize three equivalents of the alcohol: : 2 HCrO4− + 3 RR'C(OH)H + 8 H+ + 4 H2O → 2 r(H2O)6sup>3+ + 3 RR'CO For oxidation of primary alcohols to carboxylic acids, 4 equivalents of chromic acid oxidize 3 equivalents of the alcohol. The aldehyde is an intermediate. :4 HCrO4− + 3 RCH2OH + 16 H+ + 11 H2O → 4 r(H2O)6sup>3+ + 3 RCOOH The inorganic products are green, characteristic of chromium(III) aquo complexes. Like many other oxidations of alcohols by metal oxides, the reaction proceeds via the formation of a mixed chromate ester: Theseester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
s have the formula CrO3(OCH2R)−
:CrO3(OH)− + RCH2OH → CrO3(OCH2R)− + H2O
Like conventional esters, the formation of this chromate ester is accelerated by the acid. These esters can be isolated when the alcohol is tertiary because these lack the α hydrogen that would be lost to form the carbonyl. For example, using ''tert''-butyl alcohol, one can isolate ''tert''-butyl chromate ((CH3)3CO)2CrO2), which is itself a good oxidant.
For those structures with hydrogen alpha to the oxygen, the chromate esters degrade, releasing the carbonyl product and an ill-defined Cr(IV) product:
:CrO3(OCH2R)− → CrO2OH− + O=CHR
The deuterated alcohols HOCD2R oxidize about six times slower than the undeuterated derivatives. This large kinetic isotope effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for th ...
shows that the C–H (or C–D) bond breaks in the rate-determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
.
 The reaction stoichiometry implicates the Cr(IV) species "CrO2OH−", which comproportionates with the chromic acid to give a Cr(V) oxide, which also functions as an oxidant for the alcohol.
The oxidation of the aldehydes is proposed to proceed via the formation of
The reaction stoichiometry implicates the Cr(IV) species "CrO2OH−", which comproportionates with the chromic acid to give a Cr(V) oxide, which also functions as an oxidant for the alcohol.
The oxidation of the aldehydes is proposed to proceed via the formation of hemiacetal
A hemiacetal or a hemiketal has the general formula R1R2C(OH)OR, where R1 or R2 is hydrogen or an organic substituent. They generally result from the addition of an alcohol to an aldehyde or a ketone, although the latter are sometimes called hemi ...
-like intermediates, which arise from the addition of the O3CrO-H− bond across the C=O bond.
The reagent rarely oxidizes unsaturated bonds.
Illustrative reactions and applications
It remains useful in organic synthesis. A variety of spectroscopic techniques, includingInfrared spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
, can be used to monitor the progress of a Jones oxidation reaction. At one time the Jones oxidation was used in breathalyzer
A breathalyzer or breathalyser (a portmanteau of ''breath'' and ''analyzer/analyser'') is a device for estimating blood alcohol content (BAC), or to detect viruses or diseases from a breath sample.
The name is a genericized trademark of the Br ...
s.
Related processes
The principal reagents are Collins reagent, PDC, and PCC. These reagents represent improvements over inorganic chromium(VI) reagents such asJones reagent
Jones may refer to:
People
*Jones (surname), a common Welsh and English surname
*List of people with surname Jones
* Jones (singer), a British singer-songwriter
Arts and entertainment
* Jones (''Animal Farm''), a human character in George Orwell ...
.
Historical references
* * * * * * *References
{{reflist Organic oxidation reactions Name reactions